BIO 442 MENU
syllabus 
1 - genome
2 - mutate
3 -cell cycle
4 - karyotype
5 - chromoabn
6 -sex-determ
7 -prenatal
8 - mendelian
9 - complex
10 - non-trad
11 - clinical
12 - newborn
13 - teratog 
14 - linkage
15 - DNA prof 
16 - quanti 
17 - links
18 - quizzes
(full title of lecture appears in status bar on the top or at the bottom of your window)
Biology 442 - Human Genetics
Patterns of Inheritance II
Complex and Multifactorial Inheritance
Although many human disorders are inherited as single gene defects or as chromosome abnormalities, many common diseases and common congenital malformations are not. It is thought that for many disorders that several pairs of additive genes are involved. These traits are referred to as polygenic (several pairs of genes at different loci with additive effects) or multifactorial which means they are polygenic but also influenced by other genes and the pre and post natal environment. Fingerprint ridge count appears to be purely polygenic, height and skin color are multifactorial. Height is influenced by the sex of the individual and by nutrition.
Some multifactorial traits are often expressed more frequently in one or the other sex. For example, cleft lip/cleft palate is more common in males. One subgroup of multifactorial traits, such as CL/CP, exhibit a threshold effect. This refers to the hypothesis that one must have a certain number of genes for the trait to express. In the case of CL/CP female have a higher threshold and must have more genes to express the trait. Therefore, an affected female has a higher risk than an affected male of having an affected male child. All people are assumed to carry some of these genes so both parents contribute genes. The following page is a list of several traits considered at this time to be multifactorial. Neural tube defects (NTDs) are in this category. NTDs include anencephaly (lethal) and spina bifida (causes paralysis). NTDs are more frequent in Hispanics. It has been found that high levels of folate (folic acid) can reduce the risk of having a child with an NTD. It also reduces the risk of other birth defects and protects adults from heart disease.
More recently the term, oligogenic, has been used for traits with a major susceptibility locus but whose expression is also influenced by a few other genes as well as the environment. Diabetes, hypertension, cancer, schizophrenia are examples of oligogenic traits. Often the use of the terms multifactorial and oligogenic are more a reflection of our current lack of knowledge about the real genetic basis of the disorder than an accurate description of an inheritance pattern. The genetics of common disease is only recently being explored. In many of these disorders, there are major susceptibility loci but they differ in different families, Alzheimer, asthma, schizophrenia are examples. Heritability studies, complex segregation analyses, candidate gene linkage and association studies, genome-wide linkage scans and animal models are all part of the arsenal to hunt-down the susceptibility genes.
Some Empiric Risks for Common (Multifactorial) Congenital Anomalies
In general, if 2 unaffected parents have 2 affected children, the risk doubles, or if an affected parent already had an affected child, the risk also doubles.
|
Normal parents 1 affected child subsequent children |
1 affected parent risk for first child |
Identical Twin | Male/Female Ratio |
Population Incidence |
|
| Cleft lip and palate |
4% 2.5% unilateral lip 5.6% bilateral lip/palate |
3.2% | 31% | 2:1 | 1/1000 |
| whites | 1/750 | ||||
| blacks | 1/2500 | ||||
| Navajos | 1/500 | ||||
| Japanese | 1/600 | ||||
| B. C. Indians | 1/380 | ||||
| Cleft palate | 2% | 6% | 40% | 3:2 | 0.4/1000 |
| whites | 1/2000 | ||||
| blacks | 1/2500 | ||||
| Navajos | 1/2800 | ||||
| Clubfoot | 3% | 3% | 33% | 2:1 | 1.2/1000 |
| Congenital heart disease | |||||
|
VSD
|
4 - 5% | 3 - 4% | 1.3:1 | 5/1000 | |
|
PDA
|
1 - 4% | 2.8% | 1/2000 | ||
|
Tetralogy of Fallot
|
2 - 3% | 1.6% | |||
|
ASD
|
3% | 3.5%< | 1/1000 | ||
|
Pulmonary stenosis
|
<3% | 3% | |||
|
Aortic stenosis
|
3% | ||||
|
Coarctation
|
2% | ||||
|
Transposition
|
2% | ||||
|
AV Canal
|
2 - 3% | ||||
|
More complex
anomalies |
1 - 2% | ||||
| NTD spina bifida anencephaly |
2% B.C. 3% U.S.A. 5% G.B. |
2%
3% |
21% |
1/700
1/330 London |
|
| whites Jews blacks Puerto Ricans |
1/700 Boston 1/1200 1/1500 1/500 |
||||
| Congenital hip dislocation | 3.5% | 3 - 5% | 35% | 1:7 | 2/1000 |
| Pyloric stenosis | 3.2% if brother affected 6.5% if sister affected |
25.4% if mother affected 4.2% if father affected |
> males affected |
Multifactorial Inheritance and Complex Traits
“Almost all disorders in (hu)man(s) are familial in that they are more likely to afflict someone with an affected relative than someone with an equivalent set of unaffected relatives.” J H Edwards in BR Med Bull 1969;25:58-64
Multifactorial and complex traits include:
Common diseases of adult life such as diabetes mellitus, hypertension, schizophrenia, alcoholism
Normal variation in height, skin color, IQ
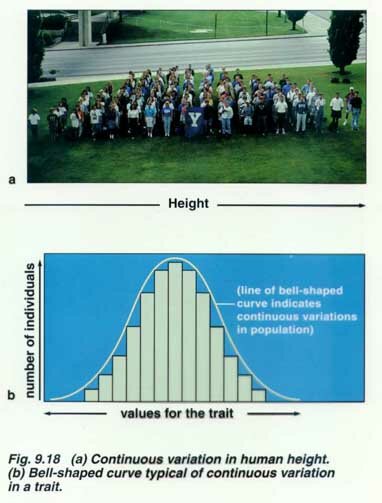
Common congenital malformations such as cleft lip, cleft palate, neural tube defects
Multifactorial and complex traits refer to
Polygenic traits caused by the impact of many different genes, each having only a limited individual impact on the phenotype
Oligogenic traits caused by effects of relatively few genes some of which have a rather large effect on the phenotype (susceptibility genes) in some cases with susceptibility genes acting in a dominant pattern with variable expressivity with the interplay of multiple environmental factors with these multiple genes.
To tease apart genetic and environmental factors of complex traits, geneticists turn to DZ and MZ twins.
For DZ and MZ twins: a trait with a greater concordance between MZ twin pairs than DZ twin pairs is at least partially controlled by heredity.
These are the results of studies of concordance rates with dizygotic and monozygotic twins
They also study MZ twins separated at birth since many of their traits held in common can be attributed to genetics, especially if their environments have been very different.
Their differences reflect the effect of environment.
Comparison of adoptees with their biological and adoptive parents provide information.
Similarities between adopted people and adoptive parents reflect environmental influences.
Similarities between adoptees and their biological parents mostly reflect genetic influences.
Examples include alcoholism studies
Alcoholism among male adoptees (table in presentation)
| Biological Parent | # in sample | % of adopted sons who were alcoholic | averages of both parents |
| Alcoholic father | 89 | 39.4 | 34% |
| Alcoholic mother | 42 | 28.6 | |
| Non alcoholic father | 723 | 13.6 | 14.6% |
| Non alcoholic mother | 1029 | 15.5 |
Polygenic Traits are quantitative rather than qualitative in nature. They are frequently distributed continuously in the population, often in a more or less bell shaped curve or they may fit the threshold model.
Polygenic traits include height, blood pressure, cleft lip ± cleft palate, NTDs .
A frequency distribution of systolic blood pressure determined by a two-locus two allele model was presented.
Also a model showing the effect of different numbers of gene loci on the phenotypic frequency distribution
The Threshold Model:
Discontinuous traits from continuously distributed liability
Recurrence Risks for Polygenic Disease or Malformation
Risks are different from Mendelian inheritance risks.
Risks represent average risks and will vary among different families.
The risk increases with the number of affected relatives.
The risk increases with the severity of the malformation or disease.
The differential risk to relatives of an affected proband increases as the frequency of the disease or malformation in the general population decreases.
When the sex ratio of affected probands deviates significantly from unity, offspring of affected probands of the less frequently affected sex are at higher relative risk.
Terminology used for relatives of the proband depends on the number of shared genes.
First-degree: parents, sibs and offspring of the proband share 50% (1/2) of their genes.
Second-degree: grandparents and grandchildren, uncles and aunts, nephews and nieces, half-sibs share 25% (1/4) of their genes.
Third-degree: first cousins, great-grandparents, great grandchildren share 12.5% (1/8) of their genes.
Proportion of Children Affected with Pyloric Stenosis
n Proband Children
Sons Daughters
Father 5.5 % 2.4%
Mother 19.4 % 7.3%
Population 0.5% 0.1%
Incidence
Family patterns in some common congenital malformations (given in presentation)
Heritability = G = G
G + B + E (total phenotypic variance) V
= variance in DZ pairs-variance in MZ pairs
variance in DZ pairs
B (family environment) E (random environmental factors)
Many multifactorial traits are influenced both by genes and environment.
The concept of heritability tries to separate their relative roles.
By definition, heritability is the proportion of the total phenotypic variance (V) of a trait that is caused by additive genetic variance.
Summary of the characteristics of multifactorial inheritance
1. Although the disorder is obviously familial, there is no distinctive pattern of inheritance within a single family.
2. The risk to first-degree relatives, determined from family studies, is approximately the square root of the population risk.
3. The risk is sharply lower for second-degree than for first-degree relatives, but it declines less rapidly for more remote relatives.
4. The recurrence risk is higher when more than one family member is affected.
5. The more severe the malformation, the greater the recurrence risk.
6. If a multifactorial trait is more frequent in one sex than in the other, the risk is higher for relatives of patients of the less susceptible sex.
7. If the concordance rate in DZ twins is less than half the rate in MZ twins, the trait cannot be autosomal dominant, and if it is less than a quarter of the MZ rate, it cannot be autosomal recessive.
8. An increased recurrence risk when the parents are consanguineous suggests that multiple factors with additive effects may be involved.
HUMAN DISEASE CATEGORIES. While these categories served practical purposes such as recurrence risk assessment, there are many overlaps among them.
Here is another group of definitions from Sheffield, V. et al. (1998) Trends Genet.14, 391-396, with some modifications by CDG.
Chromosome disorders. Disorders that result from an abnormality in the number of chromosomes, or that result from the rearrangement, duplication or deletion of large chromosomal regions.
Monogenic disorders. Diseases that result from mutation in a single gene. Complexity with this group arises from the fact that mutations in different individual genes often result in clinically indistinguishable phenotypes (locus heterogeneity). Another complexity is that of variable expressivity and incomplete penetrance. Even purely monogenic disorders can be modified by other genes and/or environmental factors. An example is PKU control by lowering phenylalanine in the diet. Mitochondrial disorders could be placed in this category.
Polygenic disorders. Diseases that arise when mutations in more than one gene contribute to the disease. The simplest are those that have a single major genetic component modified by one or a few modifier genes. An example is a susceptibility locus for congenital heart disease that was mapped to chromosome 1. The locus shows incomplete penetrance and variable expressivity indicating that other genes and/or environmental factors influence the phenotype. There can also be interaction of multiple genetic mutation, each of which contribute to the phenotype. Each mutant gene could contribute to the disorder in an additive fashion, leading to a quantitative phenotype or contribute to reaching a disease threshold.
Multifactorial disorders. Phenotypes that result from the interaction of genetic and environmental components.
Environmental disorders. Diseases that result principally from environmental influences. Even disorders of this type can have genetic susceptibility components. An example is resistance to HIV-1 infections in individuals with variant alleles of the CCR5 and CCR2 chemokine receptor genes.
Twenty-five Most Common Multiple Congenital Anomaly (MCA) Syndromes
767 Cases seen at UCSF Genetic Service 1970 -1980
| MALFORMATION SYNDROME | # OF CASES | ETIOLOGY |
| Trisomy 21 (Downs Syndrome) | 328 | Chromosomal |
| Potter Oligohydramnios Sequence | 38 | Unknown; Sporadic; recurrence risk for sibs 1-3% |
| Amniotic Band Syndrome | 34 | Unknown; Sporadic; low recurrence risk (AD/AR?) |
| Osteogenesis Imperfecta | 30 | Single Gene (AD) |
| Trisomy 18 Edward Syndrome | 30 | Chromosomal |
| VACTERL Association | 26 | Unknown; Sporadic; RR 1%; more frequent in diabetic mothers (AD/mt?) |
| Marfan Syndrome | 23 | Single Gene (AD) |
| Prader-Willi | 22 | Microdeletion; UPD; Imprinting; 1.6 % RR |
| Noonan Syndrome | 21 | Single Gene (AD) (some families); Unknown/Sporadic in others |
| Williams Syndrome | 18 | Microdeletion, Sporadic; Low RR |
| Achondroplasia | 17 | Single Gene (AD) |
| Trisomy 13 Patau Syndrome | 16 | Chromosomal |
| Turners Syndrome | 16 | Chromosomal |
| Ehlers-Danlos Syndrome | 16 | Single Gene (AD) |
| Rubinstein-Taybi Syndrome | 16 | Deletion; Sporadic; Low RR (AD?) |
| Klippel-Trenaunay-Weber Syndrome | 15 | Unknown; Sporadic; Low RR (AD?) |
| Fetal Alcohol Syndrome | 14 | Teratogen |
| de Lange Syndrome | 14 | Unknown; Sporadic; Recurrence Risk for sibs 2% (AD) |
| Moebius Syndrome | 14 | Unknown; sporadic; RR 1-2% |
| Hemifacial Microsomia (Goldenhar Syndrome) | 13 | Unknown; Sporadic; Low RR (AD?) |
| Pierre Robin Sequence | 10 | Unknown; Sporadic; Low RR (AD?) |
| CHARGE Association | 10 | Unknown; Sporadic; Low RR (AR/XL/multifactorial?) |
| Laurence-Moon-(Biedl-Bardet) Syndrome | 9 | Single Gene (AR) |
| Russel-Silver Syndrome | 9 | Unknown; Sporadic; Low RR (AD/XL) |
| Fetal Dilantin Syndrome | 9 | Teratogen |
Percentages of Cases in Major Multiple Congenital Anomalies (MCAs)by Etiologic Categories
| ETIOLOGIC CATEGORY | NUMBER OF CASES |
PERCENTAGE %
|
| Chromosomal |
428
|
66.56%
|
| Single Gene |
9
|
1.40%
|
| Teratogen |
23
|
3.58%
|
| Unknown; Sporadic (Mixture) |
183
|
28.46%
|
| Total |
767
|
100.00%
|

