BIO 102 MENU
syllabus 
1 - origin 
2 - biomol. 
3 - biomol2 
4 - viruses
5 - prokaryon 
6 - endosym 
7 - eukaryon 
8 - energy 
9 - mitosis 
10 - meiosis 
11 - reprod 
12 - genetics 
13 - humgene 
14 - humge2 
15 - evolution 
16 - evolutio2 
17 - diversity 
18 - diversi2 
19 - tissues 
20 -digestive 
21 - respirat 
22 - circul 
23 - excret 
24 - endocr 
25 - receptors 
26 - nervsys 
Quizzes 
Bio 103 Lab 
(full title of lecture appears in status bar on the top or at the bottom of your window)
Biology 102 - General Biology
Biomolecules - part 1
Sizes in Biology
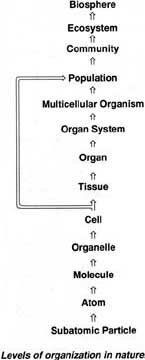
It is important to have a basic understanding of the sizes of things in our world. The smallest that we know are sub-atomic particles from there we can progress to atoms. Atoms get together to form molecules. An example is water, H2O, which is composed of two atoms of hydrogen and one of oxygen. Molecules form intracellular structures and the cells themselves. Cells are the basic unit of all organisms. There are two types, prokaryotic which are simple cells found in bacteria and blue-green algae, and eukaryotic, which are more complex and which contain organelles. In multicellular organisms, cells form tissues (example, muscle), tissues form organs (example, heart), and several organs function as an organ system (example, circulatory system). Organisms form populations, populations form communities, communities form ecosystems. The biosphere refers to all regions of the earth's waters, crust and atmosphere in which organisms live.
Universal Building Blocks of Living Things
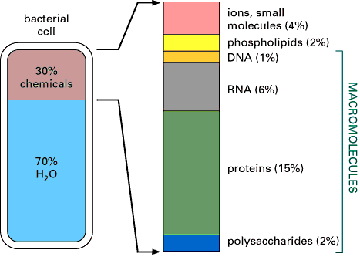
The same molecules are found in all earth organisms today. The simplest, and some of the more complex of the organic molecules found in living organisms, have been synthesized in vitro (literally, in glass, meaning in the test tube). Many experiments occurred on the primitive earth before the first simple prokaryotic cells appeared. An interesting fact is that all present day organisms are composed of the same molecules, which I will refer to as biomolecules.
"Small" "Middle Sized" and "Large" Biomolecules
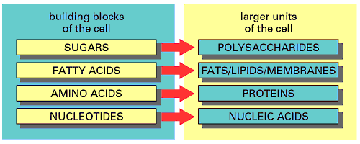
For purposes of categorizing them for you to learn, I will divide them by size or molecular weight. The smallest ones have a molecular weight of less than 100 daltons (daltons are the units of molecular weight, for example, hydrogen has a molecular weight of one dalton). The second category, the "middle-sized" biomolecules, have a molecular weight between 100 and 1000 daltons and the third category is of the "large" biomolecules, have a molecular weight over 1000 daltons. The large biomolecules are often called macromolecules because of their size. These are the proteins (polypeptides), nucleic acids (polynucleotides) and carbohydrates (polysaccharides). Lipids are medium sized but many of them have subunits like the bigger biomolecules and so they have that in common with the larger biomolecules. Both the middle-sized and macro biomolecules all contain carbon and so are called organic molecules. The subunits of the macromolecules belong in the middle-sized biomolecule group.
The Large and Middle Sized Biomolecules Are Organic Molecules
All of the biomolecules except minerals, contain carbon and as a consequence are called organic molecules. They also contain atoms of nitrogen, hydrogen, carbon, oxygen and some contain sulfate or phosphate as an integral part of the molecules. The middle-sized molecules which are between 100 and 1000 daltons are the subunits of the macromolecules, the vitamins, and "metabolites" or "intermediates." This latter group are molecules which are being degraded or synthesized. The subunits of proteins are the 20 different amino acids, the subunits of nucleic acids are the five different nucleotides (ATUCG), the subunits of many lipids are a variety of fatty acids. The subunits of carbohydrates or polysaccharides are called monosaccharides or sugars.
The Small Biomolecules: Minerals (Trace Elements)
The minerals are inorganic ions and the smallest of the biomolecules. Their molecular weight is less than 100 daltons. When one looks at the periodic table with all the known elements, we find that all or almost all are required by living organisms. This is true for both simple and complex life. Some of the minerals such as calcium and phosphate are used in larger amounts by organisms when they are used to build structures such as bones and shells (exo and endoskeletons). Also, large amounts are needed in body fluids for osmotic balance with the cells of the organism. Another very important function of minerals is to act as cofactors of enzymes. These are essential for all living systems and for this function only small amounts are necessary.
These are often called minerals and many of them are ions (charged atoms or molecules). Examples are H2O, Na+, Cl-, PO4=, Mg+, K+, Ca2+, Fe2+, and many, many more. In fact, it appears that we may need at least trace amounts of each of the elements in the periodic table. Depending on the organism, the minerals may be needed in large amounts for structural parts of the body such as bone or for body fluids, such as our blood, which needs to be isotonic with the cells it bathes. Some of the minerals are only needed in trace amounts. The function of these is to act as cofactors for proteins. Many proteins are enzymes which catalyze the myriad of reactions that occur continuously in the cells of all organisms. Enzymes usually need mineral cofactors to carry out their work. Another example is iron which is found in the protein, hemoglobin, which is in our red blood cells and which is responsible for carrying oxygen to every cell in our body. Magnesium is an integral part of chlorophyll which is the primary molecule responsible for photosynthesis in algae and plant cells. The iron and the magnesium are both held by a porphyrin ring (described in the previous lecture) within these molecules.
The Middle-Sized Molecules: Vitamins and Subunits of Macromolecules
The middle-sized molecules are organic molecules and most (the amino acids, nucleotides, sugars (monosaccharides) and fatty acids) are subunits of the macromolecules. However, some are not. The vitamins are not subunits, rather they act as cofactors (coenzymes) for enzymes and other proteins. Like the mineral cofactors, they are needed only in trace amounts. One very important middle-sized molecule is ATP (adenosine triphosphate) which is the molecule which carries the energy produced in the body. It acts as a cofactor in those chemical reactions that require energy. ATP is the common "dollar bill" of energy in all cells and organisms. There are many middle-sized organic molecules which are on their way to becoming subunits, being broken down for energy, or being formed into other metabolites for use or for excretion from our bodies. We can call this latter group, metabolites.
Vitamins (Act as Cofactors or Coenzymes)
Vitamins are medium sized biomolecules which contain carbon and are, therefore, organic molecules. They are not subunits of any macromolecule, however they function as cofactors (or coenzymes) and work with enzymes and other proteins in our cells. The vitamins are all converted into molecules called coenzymes. Folate or folic acid is a vitamin that we know is important in preventing birth defects and in protecting us from heart disease. Vitamin D deficiency causes rickets in children and softening of the bones and osteoporosis in adults. Vitamin A and E are called antioxidants and protect the cells from "free radicals" which can cause gene mutations and therefore cancer. (Cancer is caused by mutations in our body cells.) Vitamin K helps in blood clotting. A rat poison, warfarin (Wisconsin Alumni Research Foundation patented it) is an analog of Vitamin K meaning that it looks chemically like Vitamin K but does not function like Vitamin K. Rats get injured frequently and if they have ingested warfarin, they will die because of internal hemorrhaging. It is an effective and efficient poison because other rats that eat the dead rat will also get a dose of warfarin and die. Vitamin A which is synthesized from beta carotene is converted to retinene and works with a protein called opsin. Together they form rhodopsin, a very important molecule in vision. The RDA (recommended daily amount) is a minimal dose below which you will have symptoms associated with a deficiency of that vitamin. Long ago, sailors did not get enough Vitamin C because they did not have fresh fruit and vegetable on board. They got scurvy marked by anemia, spongy gums, a tendency to hemorrhages in the mouth and a hardening of the leg muscles. Vitamins do not have the exact same function in all organisms as they do in us. Many agree that it is a good idea to supplement your diet with vitamins and minerals to be certain you have what you need. Each of us is biochemically unique in our requirements for vitamins.
Informational Large Biomolecules
(More about these in a later lecture)
Proteins
Proteins are made up of linear sequences of amino acids. There are 20 naturally occurring amino acids in all living organisms. The number of different amino acids (20) is close to the number of letters in our alphabet (26). Therefore you can immediately understand that a large number of different proteins with totally different functions can be made from different permutations and combinations of these 20 amino acids strung together into "protein words." Like our words, letters can be used more than once. However, unlike our words, each protein contains hundreds of amino acids and so the number of different proteins is very large. Proteins are coded for by our genes. The genes produce the proteins which are responsible for all the work of all our cells. Many proteins are enzymes which catalyze reactions (examples are digestive enzymes in the intestine). Some proteins are structural like the collagens which make up much of our connective tissue. Some, like hemoglobin which carries oxygen, are carriers specialized to carry metabolites throughout the body. Some are hormones like insulin that control metabolism and growth. Proteins usually work with cofactors and coenzymes such as minerals and vitamins which assist them in their jobs.
Nucleic acid
Nucleic acids are made up of linear sequences of nucleotides. DNA (deoxyribose nucleic acid) is the genetic material of all cells. RNAs (ribose nucleic acid) which are closely related, are copies of the genes which are sent out to the cytoplasm of the cell to direct the synthesis of proteins for which the genes code. DNA is composed of four different nucleotides abbreviated A, T, C, and G. RNA is also composed of four nucleotides except T is replaced by U. The letters stand for the bases adenine, thymine, cytosine, guanine, and uracil which form an important part of the nucleotide. Nucleotides contain a (purine or pyrimidine) base attached to a sugar (ribose or deoxyribose) and phosphate (PO4 =). The four bases in DNA code for all our genetic information and therefore for all the proteins we make. While at first it might be difficult to understand how only four subunits can code for such a large molecule as a protein, just remember that the Morse Code which has only a dot, dash and space, codes for all the letters in our alphabet.
Both proteins and nucleic acids are called informational molecules because the sequence of their subunits is highly variable and it is the sequence which determines their function. The sequence of bases in DNA spells a very precise sequence of amino acids which makes the gene products, our proteins. The sequence is critical. This can be appreciated by the example of using the same three letters of our alphabet to spell words with entirely different meanings: EAT, TEA, ATE. Changes in the sequence of bases in DNA are called mutations and can cause serious disorders because the proteins for which they code will not be made correctly.
Non Informational Large Biomolecules
Carbohydrates or polysaccharides
Carbohydrates or polysaccharides are made up of linear and branched sequences of monosaccharides sometimes called sugars. They are usually quite monotonous repeats of the same sugar (monosaccharide) over and over. The bonds between the sugars may vary to produce polysaccharides with different properties. Glycogen is a polysaccharide we store in our liver. It has both linear and branched regions but it is composed entirely of glucose, a simple monosaccharide. Starch in the potatoes we eat is composed exclusively of glucose, also. These carbohydrates are principally a way to store energy for future use. Cellulose is also a polysaccharide composed solely of glucose, however, the bonds between the glucose molecules are different and we do not have enzymes to break them. We can eat celery, for example, which will fill our stomach but not provide many calories since we cannot break it down. Herbivores (plant eaters) such as cows and horses have to have microorganisms in their digestive tract which produce enzymes which can break the bonds. Cows have them in one of their four stomach compartments and horses have them in a caecum similar to our appendix. Mother cows have to lick their calves to transmit to them an inoculum of microorganisms for their digestive tract. Cows are much more efficient in their digestion of cellulose than are horses, as evidenced by the consistency of their feces. Cellulose is an example of a carbohydrate whose function is structural. Chitin which forms the skeleton of crabs, lobsters, etc., is also a polysaccharide. Oligosaccharides are short chains of sugars which are attached to many of our proteins and which act like zip codes, signaling other molecules to attach to them.
Carbohydrates or Polysaccharides (and Sugars)
The term carbohydrate is often used very loosely to mean both simple sugars and more complex sequences of simple sugars. The term refers to the fact that carbohydrates all contain carbon atoms which are "hydrated".........C n(H2O)n.....where "n" refers to the fact that the number can vary. Sugars (monosaccharides) usually have five or six carbons. The term, "simple carbohydrate" means one or a few monosaccharides and "complex carbohydrate," means larger sequences of monosaccharides or what biochemists call polysaccharides. Labels on canned and other foods will often refer to the "carbohydrate" content and include the amount of sugar in the product as well as complex carbohydrates. Many of the simple sugars or monosaccharides contain six carbons (glucose, fructose, galactose). The sugars that are part of nucleotides are five carbon sugars (ribose and deoxyribose). Carbohydrates and sugars usually have names ending in "ose." The sugar on your table is sucrose which is a disaccharide composed of one molecule of glucose and one molecule of fructose. Glucose is not very sweet but fructose is.
Polysaccharides include glycogen, plant starch (amylose), cellulose, and chiton (a constituent of the shells of arthropods). They are all homopolymers meaning they are all made of only one sugar repeated over and over. The first three are composed exclusively of glucose but some have branches (glycogen) and some do not (plant starch and cellulose). Cellulose is the primary component of plant cell walls. The glucose units of which it is composed are linked together by a slightly different bond which cannot be broken down by the enzymes found in animals although some microorganisms have enzymes that can digest cellulose. Therefore, herbivores must have cultures of these microorganisms somewhere in their digestive tract. Our salivary gland produces amylase, an enzyme which breaks down amylose (plant starch). (Enzyme names end in "ase.") If you eat a soda cracker and hold it in your mouth awhile it will begin to taste sweet because you are breaking the bonds of the carbohydrate into its component glucose units which have a sweet taste.
Chitin is also a homopolymer but is made of repeating subunits of N-acetyl-glucosamine, a derivative of glucose which has an amino group (containing nitrogen). We also have some extracellular polysaccharides in our joints called glycosaminoglycans (GAGs). These act as "springs" in our joints. Today chondroitin sulfate (a GAG) and glucosamine (a monosaccharide constituent) are sold in health food stores to prevent or ameliorate arthritis. Some lethal genetic disorders are due to the lack of the appropriate enzyme needed to recycle these GAGs which then accumulate in the joints and in openings in bone.
Oligo means few. (Poly means many.) Oligosaccharides are a few sugars attached to one another. They are often attached to proteins and lipids found in the cell membrane. The protein is then called a glycoprotein and the lipid is called a glycolipid. They are signals, sort of like "zip codes." They are recognition sites for molecules (hormones), foreign organisms (bacteria, viruses) and other cells. The A, B, O, AB blood types are due to glycoproteins and glycolipids on our cell membranes. The genes for the blood types code for enzymes which synthesize the oligosaccharide portion of a glycoprotein and glycolipid in the cell membrane.
Oligosaccharides attached to lipid molecules. The ABO blood groups in humans are due to these oligosaccharides on cell membranes.
Lipids: Fats and Sterols
Lipids are the best energy storage molecules for their weight. The breakdown of fatty acids produces both energy and metabolic water, hence the camel stores lipids in his/her hump. Waxes contain fatty acids, also. Sterols are another kind of lipid. Cholesterol is a lipid most of us know about. Lipids are very important in forming the membrane of the cell. The lipids are all molecules which are insoluble in water and as such are perfect for forming cell membranes which must separate one fluid containing compartment from another. Some sterols are hormones such as cortisol, testosterone, estrogen, and progesterone. These molecules are chemical messengers which enter cells and turn on specific chemical reactions.
Fatty acids which are subunits of many lipids consist of long chains of carbon and hydrogen. The number of carbons in the chain varies but is always a multiple of two. Fatty acids are "saturated" if all of the carbons in the chain have the maximum numbers of hydrogens attached (two per carbon) and as "unsaturated" when some carbons have only one hydrogen attached. Carbon atoms that only have one hydrogen form a "double bond" with the next carbon. This puts a kink in the chain and cell membranes with more unsaturated fatty acids are more fluid than those with less. Deep sea fish that live in very cold waters put unsaturated fatty acids in their membrane lipids as a kind of "antifreeze." A major function of lipids is to form the cell membrane. The lipids in the cell membrane contain fatty acids which are attached to another molecule which has charged groups on it. The part of the lipid which is charged (polar end) dissolves in the extracellular or intracellular fluid while the fatty acid chains form the inner portion of the membrane and exclude water. Some fatty acids are attached to glycerol and the resulting molecules are called triglycerides. These neutral fats function to store energy. Fats also provide insulation for the body. Waxes are lipids that consist of a long chain fatty acid attached to an alcohol. They make surfaces waterproof on such things as leaves and beeswax.
The "saponifiable lipids" contain fatty acids. Saponifiable refers to the making of soap from animal fats using lye (sodium hydroxide) to break down the lipids to their component parts. The sodium salts of the fatty acids were soap. Triglycerides are three fatty acids attached to a glycerol (glycerine) molecule. Fatty acids are long carbon chains with hydrogen attached to the carbon but no oxygen (as you see in sugars). They do not form rings. Triglycerides are stored for energy and metabolic water (as in the camel's hump). Other fatty acids attached to fancier molecules than glycerol (ones with a positive and/or negative charged group on them) have a polar (hydrophilic) head end and a non-polar (hydrophobic) tail where the fatty acids are. These lipids form a lipid bilayer and are an integral part of all cell membranes. The outer charged part of the molecules in the bilayer can interact with the extracellular fluid on one side and with the intracellular fluid on the other side of the membrane while the inner uncharged part of the molecule makes a seal so that the intra and extracellular fluid areas cannot mix easily.
Omega-3 and Omega-6 fatty acids found in fish oils
They are important in a variety of functions involving cell membranes, vision, memory, gene expression, inflammatory response
Another group of lipids are the sterols. They contain carbons in rings and not in chains. They do not contain fatty acids and thus are non-saponifiable lipids. Instead they are all built on a similar carbon ring structure that resembles a section of "chicken wire."Cholesterol is an important sterol. It serves an important function in eukaryotic cell membranes, providing flexibility. Prokaryotic cells do not contain cholesterol. Other sterols you are familiar with are the hormones, testosterone, estrogen, progesterone and cortisone. Because these hormones are fat soluble, they can enter the cell easily and do not require special receptors as the protein hormones do. Common endings for sterols are "ol" and "one."
Steroid Estridiol Testosterone

